MISSION
The Vectorology core facility produces batches of highly concentrated (109 TU / mL average) lentiviral gene transfer vectors of controlled quality at small and large scale. The laboratory staff has developed an important collection of lentiviral expression backbones ready to accept any gene of interest in order to provide the best solution for every in vitro or in vivo application encompassing basic research, gene therapy, cellular therapy and vaccine. The BSL2 and BSL3 confined zones allow to satisfy every demand of viral production. The close collaboration between iVector and the biotechnology & biotherapy team ensures the constant evolution of gene transfer technologies. iVector is part of the ICM core facility network (genotyping, histology, microscopy, cellular culture…) which facilitates translational research projects.
SERVICES
The iVector platform is specialized in the manufacture of viral vectors for gene transfer. We put our expertise and experience at your disposal to best respond to all types of applications (in vitro or in vivo) encompassing basic research, gene therapy and cell therapy. Our expertise and infrastructure (BSL2 and BSL3) allow us to produce highly concentrated lentiviral vectors and AAVs to meet all types of needs.
- Plasmid construction
- Construction and production of lentiviral vectors
- Construction and production of AAV vectors
- Provision of equipment

The activities of the Vectorology platform have been certified since 2015 regarding the ISO9001 standard (out of equipments provision activity), allowing to guarantee the traceability, the reliability and the rigour of the implemented procedures.
EXAMPLES OF APPLICATION
AAV production : VEGF-C promotes brain-derived fluid drainage, confers neuroprotection, and improves stroke outcomes.
Upon request for a project from Jean-Léon THOMAS (Research Director) and his team, the iVector platform was in charge of the construction. It was a multi-stage cloning with fragment of interest isolation (gene expressing VEGF-C) and integration into an AAV vector for expression in a target region.The next step is the production of the final viral vector.
These same vectors were injected in mice in order to study the consequences of an overexpression of VEGF-C on the drainage of cerebral fluid (see below).
*Vascular endothelial growth factor C (VEGF-C) : promote the growth of lymphatic vessels.
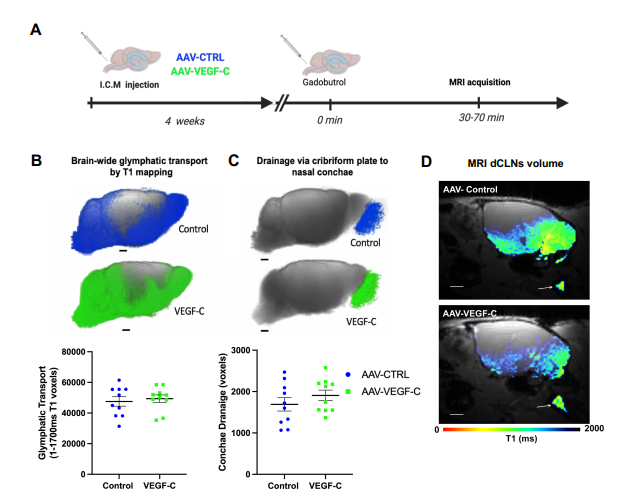
A: A: Eight weeks old mice were analyzed by DCE-MRI (Dynamic Contrast Enhanced) after a prior intracisterna magna injection of either AAV-VEGF-C or AAV- control (VEGFR34–7-Ig) at 4 weeks old.
B and C: Comparison of glymphatic transport by evaluation of T1 values corresponding to the absorption of the contrast medium (Figure B) and drainage of this contrast medium (Figure C) between control and VEGF-C mice. Both groups of mice showed a similar output of contrast medium to the nasal cavity.
D: Evaluation of contrast agent drainage of deep cervical lymph nodes (dCLN). View of sagittal plane to brain at dCLN showing T1.
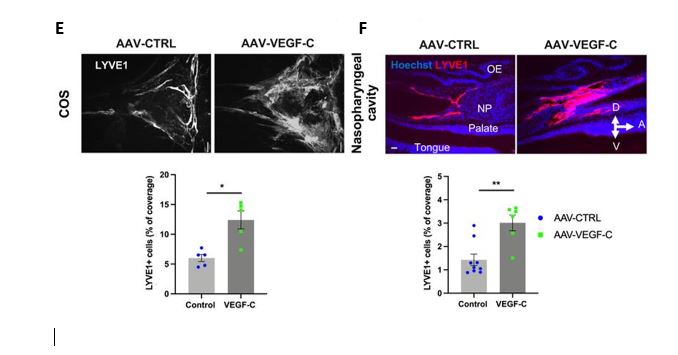
E and F : Representative images of extracranial lymphatics in the nasopharyngeal region. A: Anterior, D: dorsal, V: ventral. NP: nasopharynx, OE: olfactory epithelium. LYVE1 : Lymphatic vessel endothelial hyaluronan receptor 1.
As expected, Intra-cisternal delivery of VEGF-C was found to increase the coverage and diameter of meningeal lymphatics as expected (Fig.E), but it also remodeled extracranial nasopharyngeal lymphatics (Fig.F).
Therefore, intra-CSF VEGF-C expands the meningeal, extracranial, and lymph nodes (LN) compartments of the dura mater-to-LNs pathway.
Lentiviral production : Upon request for a project from Rana SALAM of the Emmanuelle HUILLARD, the iVector platform was in charge of the construction of plamsids (insertion of the miR-NRF2 sequence or miR-control in an H-RasV12-shp53-(IRES)-GFP vector by Gibson Assembly). The next step was the production of lentiviral vectors. NRF2 is a transcription factor. It has a protective role against oxidative stress. The project’s aim is to study the consequences of NRF2 inactivation in malignant cells on cell senescence. This study is part of an article published in January 2023 in Nature Communications: Cellular senescence in malignant cells promotes tumor progression in mouse and patient Glioblastoma (GBM).
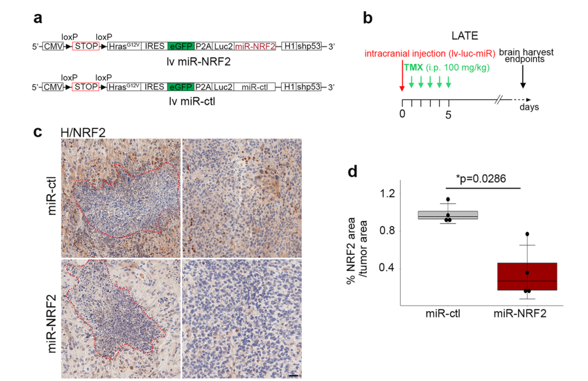
A knockdown approach was used by introducing a microRNA targeting NRF2 (miR-NRF2). miR (microRNA) are capable of gene expression extinction.
a. Scheme of the lentiviral vector containing either a miR-NRF2 or a miR-ctl. Scheme of the lentiviral vector containing either a miR-NRF2 or a miR-ctl.
b. Timeline of the mouse GBM generation at the late timepoint
c. Representative NRF2 IHC staining (brown) on miR-ctl (n = 4) and miR-NRF2 (n = 4) GBM cryosections. Necrotic areas are outlined in red dashed lines.
d. Quantification of the NRF2 area (IHC) over the tumor area.
Quantification of NRF2 by immunohistochemistry revealed a significant decrease of the protein in miR-NRF2-GBMs compared to miR-controls (ctl)-GBMs.
Contact
Email: ivector@icm-institute.org

